Autoprobiotics in the treatment of colorectal cancer
Colorectal cancer (CRC) is the second leading cause of cancer-related death worldwide. In 2020, there will be more than 1.9 million new cases of the disease, of which 930,000 will be fatal. By 2040, the number of CRC cases is estimated to increase by 63%. Conservative treatment for CRC usually involves a combination of cytostatic agents, particularly fluorouracil, leucovorin and oxaliplatin (FOLFOX), which is associated with symptoms of dyspepsia, mucositis and changes in the gut microbiome.
Scientists at the Personalized Microbial Therapy Research Laboratory of the WCRC for Personalized Medicine studied the effect of FOLFOX on the microbiota of CRC patients in vitro and the feasibility of using autoprobiotic (indigenous non-pathogenic) Enterococcus faecium in the combination therapy of CRC with FOLFOX.
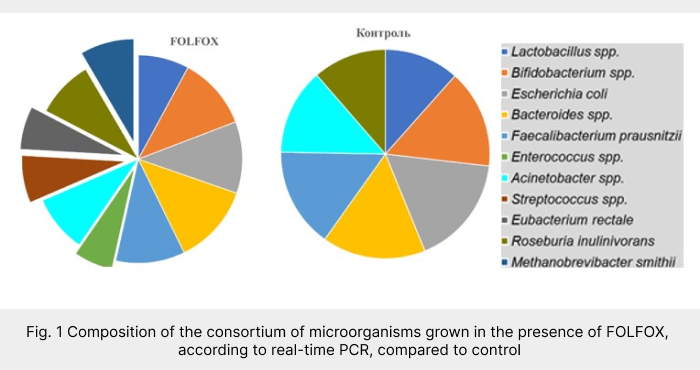
The study showed that culturing faecal samples from CRC patients prior to therapy in the presence of FOLFOX resulted in an increase in α-diversity, Klebsiella spp, atypical E. coli, Enterobacter spp, E. faecalis, Streptococcus spp, Bacteroides fragilis, Methanobrevivacter sp, Roseburia sp and Eubacterium sp, with a decrease in E. faecium (Fig. 1). Therefore, any potential changes in the gut microbiota of patients under the effect of FOLFOX can be considered unfavourable, as conditions are created that are particularly conducive to the selection of opportunistic microorganisms.
In a clinical study following the introduction of autoprobiotic E. faecium (Fig. 2) during chemotherapy in CRC patients, there was no chemotherapeutic toxicity of 3-4 grades and no development of severe mucositis. In the gut microbiota study, there was a reduction in the levels of E. faecalis, which often has collagenase activity, leading to post-operative anastomotic failure, and a reduction in the number of other opportunistic microorganisms.

The studies carried out by WCRC scientists thus demonstrate the feasibility of using autoprobiotic enterococci in the chemotherapy of colorectal cancer.
30.12.2023
