Study of cardiotoxicity from immune checkpoint inhibitors
One of the areas addressed by the Cardio-Oncology Research Group of the Centre for Personalized Oncology of the WCRC for Personalized Medicine is to identify a risk group for cardiotoxic effects of antitumor therapy in cancer patients.
To date, the mechanisms of cardiovascular complications have been well studied for anthracyclines. The spectrum of complications induced by these anticancer drugs includes progressive heart failure and rhythm and conduction disturbances in the so-called anthracycline cardiomyopathy, which requires the use of developed early detection algorithms and in some cases even the withdrawal of chemotherapy drugs.
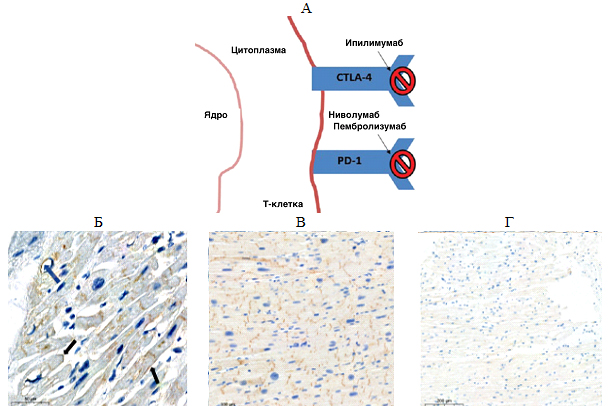
Immune checkpoint inhibitors (ICTs) are relatively new anticancer drugs. They can restart the antitumor immune response and induce tumor cell death by various signaling molecules (Fig. A).
Fig. 1. А — mechanism of action of immune checkpoint inhibitors: Cytotoxic T lymphocyte antigen-4 (CTLA-4); programmed cell death protein (PD-1) and its ligand (PD-L1); Б, В, Г — expression of PD-L1 in the myocardium of various patients: Б — patients with coronary artery disease (blue arrow – PD-L1 expression in endothelial cells and cardiomyocyte membrane; black arrow — PD-L1 expression on intercalated discs), В — patients with nonischemic dilated cardiomyopathy, Г — control group: cancer patients without cardiac complications during therapy with immune checkpoint inhibitors.
Specialists of the Cardio-Oncology Research Group are conducting basic research of the mechanisms underlying the cardiotoxic effect of immune checkpoint inhibitors causing heart damage. The first data confirming the hypothesis of the scientists of the Centre for Personalized Medicine have already been obtained. For the first time, an increase in the level of specific proteins (PD-L1) was found in the myocardium of patients with various heart diseases. A clear negative relationship was shown between the amount of PD-L1 in the heart muscle and the key indicator of cardiac performance, namely the left ventricular ejection fraction, that is, the higher the expression of the PD-L1 ligand, the lower the pumping function of the heart in the studied group. These data support the assumption that patients with initially high levels of PD-L1 are at higher risk of cardiac complications of anticancer therapy.
This study will help scientists understand the fundamental mechanisms of heart damage in cancer patients and allow safe antitumor therapy regimens.
19.04.2022
